Wednesday, July 28, 2010
Tuesday, July 27, 2010
Other products in the market for arsenic detection and monitoring
Monday, July 26, 2010
Surface Plasmon Resonance (SPR)

A light source will shone on the prism with a sensor chip attached on its surface, buffer will then flow through the prism .Hence, reflecting the light onto the mirror in a critical angle, where readings are taken down

As compared to other analysis technologies, SPR can detect a smaller quantity of arsenic compound , making it more precise then other detection method,but high cost for this technology has offset its benefits.
Animation of SPR
Arsenic detection with gold nanoparticles
Arsenic detection with gold nanoparticles works with the aggregation of gold nanoparticles, and it selectively detects arsenic in drinking water down to concentrations of 3 ppt (parts per trillion).
Countries like India, Bangladesh, and Thailand are primarily affected by ground water with high arsenic concentrations. However, high concentrations of arsenic have also been found in some areas of North and South America. Once detected, the problem can fairly easily be addressed.
Current analytical techniques are time-consuming and require a series of enrichment steps.
The new process could now speed up and simplify arsenic analysis.
Special organic molecules were to the surfaces of the gold nanoparticles. These molecules act as “ligands” for arsenic, meaning that they form a complex with it.
Each arsenic ion can bind to three ligands, which allows it to link together up to three gold particles.
The higher the arsenic concentration in the sample, the more strongly the gold particles clump together and the number of bigger aggregates increases.
The color of gold nanoparticles in a liquid depends on their size. Whereas the arsenic-free gold nano-particles appear red, arsenic-induced aggregation causes the color to change to blue.
Concentrations down to 1 ppb can be detected with the naked eye by means of the color change. Arsenic binds to the ligands much more strongly than other metals; the researchers were able to increase this selectivity by attaching three different ligands to the gold.
One very precise method for detecting minimal changes in particle size is dynamic light scattering (DLS), in which laser light scattered by the particles is analyzed. By using DLS, Ray and his co-workers were able to detect and quantify arsenic concentrations as low as 3 ppt. In samples of well water from Bangladesh, the team found 28 ppb arsenic; in water from taps in Jackson (Mississippi, USA) they found 380 ppt.
Sunday, July 25, 2010
References
www.norwich.edu/about/news/2008/...ter.html
http://www.geocities.jp/senribb/jewels/
torlan.ru/ccontent/update002/
www.exceptionalminerals.com/sale...om16.htm
gurumia.com/tag/dig-tube-wells/
www.h2oplusomething.com/index.ph...mid%3D65
www.niton.com/mining.aspx%3Fsflang%3Den
www.directindustry.com/prod/inno...980.html
www.directindustry.com/prod/ther...658.html
www.wichitech.com/blankettestsystem.html
www.sciencegl.com/gis_dem/index.html
elaiter.en.made-in-china.com/pro...ent.html
www.flickr.com/photos/21182585%4...4729953/
www.hcti.com/sm/aboutro/aboutro.html
theceramicengineeringblog.blogsp...ion.html
www.rigaku.com/xrf/nanohunter-show.html
international.usgs.gov/projects/...enic.htm
www.wired.com/wiredscience/2008/...prize-h
www.ornl.gov/sci/casd/etv/mti.htm
scipeeps.com/groundwater-polluti...vent-it
search.biztrademarket.com/Search...Bfurnace
www.idwr.idaho.gov/WaterInformat...enic.htm
steveaoki.dimmak.com/blog/2009/1...acteria/
www.himfr.com/d-p112896133871744...nalyzer
post.queensu.ca/~pearl/maritimes...os2.html
www.patagoniavolunteer.org/volun...nts.html
www.columbia.edu/~tsn2102/web-pa...rds.html
Reference:
http://www.bdix.net/sdnbd_org/world_env_day/2002/current_issues/arsenic.htm
http://www.terradaily.com/reports/Bangladeshi_People_Can_Help_Combat_Arsenic_Poisoning_Researchers.html
http://www.drkaslow.com/html/arsenic.html
http://www.greenfacts.org/en/arsenic/l-3/arsenic-1.htm#0p0
http://www.physics.harvard.edu/~wilson/arsenic/measurement/EPA_review.pdf
www.rsc.org/chemistryworld/News/...0901.asp
http://en.wikipedia.org/wiki/X-ray_fluorescence
http://www.asaanalytics.com/asv.php
http://en.wikipedia.org/wiki/Anodic_stripping_voltammetry
http://dept.lamar.edu/engineering/COE/MIC/electrochemical_analysis.html
http://www.wagtech.co.uk/products/water-and-environmental/water-test-kits/arsenator%C2%AE-digital-arsenic-test-kit
http://www.supply.unicef.dk/Catalogue/bulletin8.htm
http://en.wikipedia.org/wiki/Arsenic
http://cleantech.com/news/3387/microbe-spots-arsenic-contamination
http://www.scientificamerican.com/article.cfm?id=using-a-poison-to-turn-sunlight-into-food
http://www.angelfire.com/ak/medinet/file9.html
http://www.sickkids.ca/PGPR/Symposia-and-Workshops/Oct-2007-china/arsenic-pollution/index.html
Saturday, July 24, 2010
Anodic Stripping Voltammetry (ASV)
ASV is also suitable for measuring dissolved arsenic in drinking water and it is equally sensitive towards As (III) and As (V).
Although ASV can be used to monitor other kinds of elements, we will now only discuss on how it detects arsenic. Basically, it works based on the principle of electroplating arsenic onto an electrode, which concentrates it. The arsenic that is electroplated or reduced onto the electrode is then stripped off or oxidized off. We can control this electroplating and stripping off action by raising or lowering the potential, which will be discussed in detailed in the procedures. The stripping off action generates a current that can be measured. The current (milliamps) is proportional to the amount of arsenic being stripped off.
Anodic stripping voltammetry usually incorporates three electrodes, a working electrode, auxiliary electrode (sometimes called the counter electrode), and reference electrode. An electrolyte is usually essential for most samples. For most standard tests, the working electrode is a mercury film electrode. The mercury film forms an amalgam(mixture) with the analyte(the substance or sample being analyzed) of interest, which upon oxidation results in a sharp peak, improving resolution between analytes. The mercury film is formed over a glassy carbon electrode. A mercury drop electrode has also been used for much the same reasons. In cases where the analyte of interest has an oxidizing potential above that of mercury, or where a mercury electrode would be otherwise unsuitable, as the analyte will not be stripped off easily as it cannot be easily oxidized. Hence, we can solve this problem by using a solid, inert metal such as silver, gold, or platinum may also be used.

The detailed procedures are shown as below:
1. The solution is continuously stirred during the first 2 steps. The first step is the cleaning step where the potential is raised to a higher potential for a period of time to fully strip the metal off from the electrode.
2. The potential is then lowered to a lower potential so as to reduce the metal and deposit it on the electrode. After this second step, the stirring is then stopped.
3. If a mercury electrode is used, more time should be allocated to make sure that the deposited material is distributed evenly onto the electrode. If a solid inert electrode is used, this step may be skipped.
4. Lastly, the working electrode is then raised to a higher potential and the metal is stripped off or oxidized. This stripping action will give off electrons, which is a measure of the current.

Advantages:
-The instrument is portable, lightweight, and field ready with long battery life (up to 40hours)
-good detection limit (0.1µg/L) as it can measure arsenic at low levels
Disadvantages:
-requires the hands of a professional to operate some operations
-high degree of instrument maintenance-instrumentation required is relatively expensive to purchase ($30,000)
-not approved by EPA as an acceptable analytical technique for measuring arsenic concentrations in drinking water
-results are interfered by the presence of other elements such as copper, mercury and zinc.

Nano-Band Explorer : an electrochemical analyzer capable of performing Anodic Stripping Voltammetry (ASV)
Conclusion
Friday, July 23, 2010
Removal of arsenic
If however, arsenite is predominant in the water sample, it can be oxidized into arsenate by pre-oxidation to arsenate. Some of the oxidizing agents that we can use include chlorine, ferric chloride, and potassium permanganate. Another alternative is by using ozone and hydrogen peroxide, but they might not be as effective as no data are available on performance.
3. using water from other sources such as rivers, rainfalls or ponds.
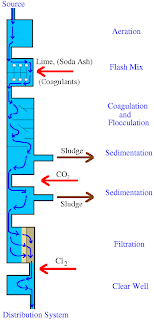
Coagulation/Filtration (C/F) is an effective treatment process for the removal of arsenic. The efficiency of this process is affected by pH as the efficiency will be reduced when the pH becomes too high or too low. One main cause of concern is that the nearby landfills may not be willing to accept this arsenic-contaminated coagulation sludge for disposal. Hence, disposal of sludge may be a problem. Well trained operators may be required for this process. This process is also very costly and the process performance may vary.
Lime Softening (LS) provides a high efficiency for arsenic removal under certain conditions. It has to be conducted at a pH of greater than 10.5 provided that the influent concentration is 50 µg/L. However, in order to further reduce the concentration of the pollutant, it may require a secondary treatment process. The following diagram shows the process of lime softening.
Activated Alumina(AA) is effective for treating water that has a high concentration of Total Suspended Solids(TDS). However, other elements such as selenium, fluoride, chloride, and sulphate, when present at high concentrations, may interfere with the removal process. This removal process is highly selective towards arsenic. One cause of concern that it may not be efficient in the long term, as it loses its adsorptive capacity with time. As this process releases highly concentrated waste streams, disposal may be a problem as well.
Ion Exchange (IE) is effective for removing arsenic. However, sulfate, TDS, selenium, fluoride, and nitrate can also interfere with the removal process. Systems containing high levels of these constituents may require pretreatment. As this process produces highly concentrated waste by-product stream, disposal may be a problem as well. The following diagram shows the process of ion exchange.

Reverse Osmosis (RO) is able to remove as much as 95% of arsenic when the right amount of operating pressure is applied. This removal process may not be suitable to be used in water-scarce regions due to water rejection.(about 20-25% of influent).
Electrodialysis Reversal (EDR) is able to achieve a removal efficiency of 80%. Studies have shown that this process can reduce an influent concentration of 21 µg/L to only 3 µg/L, which shows a decrease in 19 µg/L of arsenic concentration. Similar to reverse osmosis, there is water rejection as well. Compared to RO, this process may not be cost competitive and as efficient. However, it is easier to operate. The following diagram shows a diagram of the EDR module.
Nanofiltration (NF) can achieve a removal efficiency of over 90%. However, this method of removal might not be suitable for regions where water is scarce as the water from the influent may be rejected by as much as 25%.

Recent breakthroughs of arsenic removal
Instead of using the conventional man-made techniques to remove arsenic as mentioned above, we can now use bacteria to remove arsenic. Scientists have recently discovered a new kind of microbe that posses the ability to use this poison to turn sunlight into food. In other words, it uses arsenic to power photosynthesis, which is a process whereby plants and bacteria convert sunlight into food. This bacteria is found in a hot spring in California as they have the ability to thrive in hot temperatures. However, the bacteria can only perform this removal in the presence of sunlight, which is a basic requirement for the process of photosynthesis.

The red slime mat shown in this picture is made up of the bacteria that uses arsenic to power photosynthesis.
Tuesday, July 20, 2010
What are the sources?
Contaminated food [Seafoods]
Ground water used for drinking water supply
volcanic action
Low temperature volatilization
Man-made sources

• medications
• ore smelting/refining/processing plants, galvanizing, etching and plating processes
• burning of fossil fuels especially in coal-fired power generation plants
• Tailings from or river bottoms near gold mining areas (past or present)
• Agricultural chemicals: Insecticides, rodenticides and fungicides
• Commercial arsenic products which include: sodium arsenite, calcium arsenate, and lead arsenate.
• "Paris green" (cupric acetoarsenite) a wood preservative.
• Burning of vegetation

A global phenomena

Many other countries and districts in South East Asia, such as Vietnam, Cambodia, and China have geological environments conducive to generation of high-arsenic groundwater.


Thursday, July 15, 2010
Portable X-ray Fluorescence


X-ray fluorescence (XRF) is the emission of characteristic "secondary" (or fluorescent) X-rays from a material that has been excited by bombarding with high-energy X-rays or gamma rays. Besides measuring arsenic, it can be used to analyse other elements and chemicals like the investigation of metals, glass, ceramics, and building materials and for research in geochemistry, forensic science and archaeology. The following diagram shows the basic working principle of this process.

The amount of photon emitted will help us determine the concentration of the arsenic in the sample. A photon is a discrete bundle of light energy.
Portable X-ray fluorescence is used to measure arsenic in dry solid samples, such as soil and dried sludge. The main interferents listed in this method were variations in particle size, moisture, and lead co-contamination.
Advantages:
-Measuring devices are normally portable
- It can directly measure arsenic in the soil without having to extract the soil from the ground.
- Can measure a wide variety of metals besides arsenic
- Flexible as it can be used for measuring arsenic in both liquid and solid samples.
Disadvantages:
-It is not suitable for the detection of low concentrations of arsenic especially in drinking water as detection is only accurate at gram per litre concentrations.
-results can be interfered when lead is present in the sample
-many models contain a radioactive source, which may cause health effects to the user if not properly handled. However, research efforts have proven that this radioactive source can be eliminated and replaced by a less harmful source.
The Wagtech Arsenator system
The complete system comes with sufficient reagents and consumables for over 400 tests. The following are the advantages of using the Arsenator:
• Low cost digital arsenic testing device
• Fully portable, designed specially for field use
• Immediate results in the field in less than 20 minutes
• Simple, safe and easy to operate
• Gives accurate test results between the critical range of 2µgl (ppb) to 100µgl (ppb)
• Designed in conjunction with Prof. Walter Kosmus and laboratory tested by Imperial College London
• Field tested in conjunction with UNICEF/WHO WAT/SAN monitoring programmes
• Environmentally friendly
Colorimetric Test Kits
The following illustrates the procedures of the “Gutzeit” method :
1. treat the water sample with a reducing agent that transforms the arsenic compounds present in the water into arsenic trihydride (arsine gas).
2. Arsenic is separated from the sample
3. The arsenic trihydride diffuses out of the sample where it is exposed to a paper impregnated with mercuric bromide.
AsH3 + 3HgBr2 → As(HgBr)3 + 3HBr
The white mercury(II) bromide will turn yellow, brown, or black if arsenic is present in the sample
4. The reaction with the paper produces a highly colored compound.
5. The concentration of the arsenic can be approximated using a calibrated color scale.
This method of detection has several pros and cons as shown below.
Advantages:
- inexpensive
- minimally trained personnel can readily perform it and read the results in the field.
Disadvantage:
- sulfur, selenium, and tellurium compounds have the potential of interfering with this assay.
Using Bacteria and Plants for Arsenic Detection
 Instead of using conventional man-made technologies, can you all imagine using bacteria and plants for arsenic detection?Let's take a look at this innovative bio-technology that has been developed.
Instead of using conventional man-made technologies, can you all imagine using bacteria and plants for arsenic detection?Let's take a look at this innovative bio-technology that has been developed.
One of the advantages of biological monitoring is the fact that there is a close association between biomonitors with the biological systems under study as it the biomonitor is normally part of the biological system.

-the organism must be capable of accumulating metals in measurable amounts.
-the organism, or relevant parts of it, must be readily available both in terms of quantity and distribution so that unbiased sampling is possible.
-it should be available throughout the year or throughout the period of study, with relative ease of collection.
-Upon exposure, the organism should show a differential uptake or accumulation which allows us to determine the relative pollution levels and establish a relationship to deposition rate or air concentrations.
-the organism should not ingest metals from other sources, this is especially important for assesing airborne contamination.
-repeatability is essential
-reasonable cost of collection and analysis

Research has shown that bacteria can be used as a biosensor to identify and treat sites that are contaminated with arsenic. Although bacteria has been used to monitor nitrates in the past, the use of microbes to treat arsenic may also be feasible, according to scientists.
All cell-based organisms have the ability to detoxify arsenic compounds. The process involves a wide variety of proteins that will modify, transport and extrude the arsenic from the cell. In the presence of arsenic and through specific genetic mechanisms, the correct sequence of proteins can be synthesised and activated. Hence, the required proteins can be synthesized to activate the arsenic detoxification system.
Genetically modified microbes were used in another recent study to develop a set of semi-quantitative assays for potable water. The investigators also developed an assay that produced a visible blue color with arsenite concentrations above 8 ppb.
Advantages:
- can detect arsenic down to ppb levels, in other words, it can measure only small concentrations of arsenic
-good potential for assaying arsenic
-environmentally friendly
Disadvantages:
-apply only to water assays
- limited success rate
- it is not clear whether the microbes are measuring all of the arsenic in a sample or just the bioavailable arsenic.

Compared with the use of microbes for arsenic detection, there are far less research involving the use of plants to detect arsenic. A recent experiment has been conducted on two water plants upon exposure to arsenic. It is found that there is a change in the colour pigments of the plants. In order to acquire more accurate results, it requires an incubation period of three days and quantified with a series of standards. Although this is a very sustainable and "low tech" assay, the results may be affected by other factors such as nutrient levels or microbial infection, which can generate the same pigment change as arsenic absorption.
Advantages:
-its general ubiquity. Only in situations of extreme aerial contamination is vegetation likely to be sufficiently scarce to cause sampling problems. In other words, this detection method has a low risk for sampling problems to occur.
Disadvantages:
- coloration changes in plant systems may be due to factors other than arsenic detection.
-samples may vary between general herbage of several species to leaves, whole leafy shoots and bark of single species.
For more information ,feel free to read up more on : http://www.technologyreview.com/Biotech/18103/