If however, arsenite is predominant in the water sample, it can be oxidized into arsenate by pre-oxidation to arsenate. Some of the oxidizing agents that we can use include chlorine, ferric chloride, and potassium permanganate. Another alternative is by using ozone and hydrogen peroxide, but they might not be as effective as no data are available on performance.
3. using water from other sources such as rivers, rainfalls or ponds.
Coagulation/Filtration (C/F) is an effective treatment process for the removal of arsenic. The efficiency of this process is affected by pH as the efficiency will be reduced when the pH becomes too high or too low. One main cause of concern is that the nearby landfills may not be willing to accept this arsenic-contaminated coagulation sludge for disposal. Hence, disposal of sludge may be a problem. Well trained operators may be required for this process. This process is also very costly and the process performance may vary.
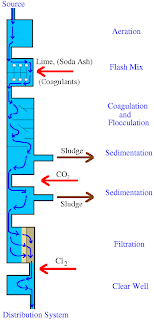
Lime Softening (LS) provides a high efficiency for arsenic removal under certain conditions. It has to be conducted at a pH of greater than 10.5 provided that the influent concentration is 50 µg/L. However, in order to further reduce the concentration of the pollutant, it may require a secondary treatment process. The following diagram shows the process of lime softening.
Activated Alumina(AA) is effective for treating water that has a high concentration of Total Suspended Solids(TDS). However, other elements such as selenium, fluoride, chloride, and sulphate, when present at high concentrations, may interfere with the removal process. This removal process is highly selective towards arsenic. One cause of concern that it may not be efficient in the long term, as it loses its adsorptive capacity with time. As this process releases highly concentrated waste streams, disposal may be a problem as well.
Ion Exchange (IE) is effective for removing arsenic. However, sulfate, TDS, selenium, fluoride, and nitrate can also interfere with the removal process. Systems containing high levels of these constituents may require pretreatment. As this process produces highly concentrated waste by-product stream, disposal may be a problem as well. The following diagram shows the process of ion exchange.

Reverse Osmosis (RO) is able to remove as much as 95% of arsenic when the right amount of operating pressure is applied. This removal process may not be suitable to be used in water-scarce regions due to water rejection.(about 20-25% of influent).
Electrodialysis Reversal (EDR) is able to achieve a removal efficiency of 80%. Studies have shown that this process can reduce an influent concentration of 21 µg/L to only 3 µg/L, which shows a decrease in 19 µg/L of arsenic concentration. Similar to reverse osmosis, there is water rejection as well. Compared to RO, this process may not be cost competitive and as efficient. However, it is easier to operate. The following diagram shows a diagram of the EDR module.
Nanofiltration (NF) can achieve a removal efficiency of over 90%. However, this method of removal might not be suitable for regions where water is scarce as the water from the influent may be rejected by as much as 25%.

Recent breakthroughs of arsenic removal
Instead of using the conventional man-made techniques to remove arsenic as mentioned above, we can now use bacteria to remove arsenic. Scientists have recently discovered a new kind of microbe that posses the ability to use this poison to turn sunlight into food. In other words, it uses arsenic to power photosynthesis, which is a process whereby plants and bacteria convert sunlight into food. This bacteria is found in a hot spring in California as they have the ability to thrive in hot temperatures. However, the bacteria can only perform this removal in the presence of sunlight, which is a basic requirement for the process of photosynthesis.

The red slime mat shown in this picture is made up of the bacteria that uses arsenic to power photosynthesis.